Syringe Label – Tamper-Evident Wrap
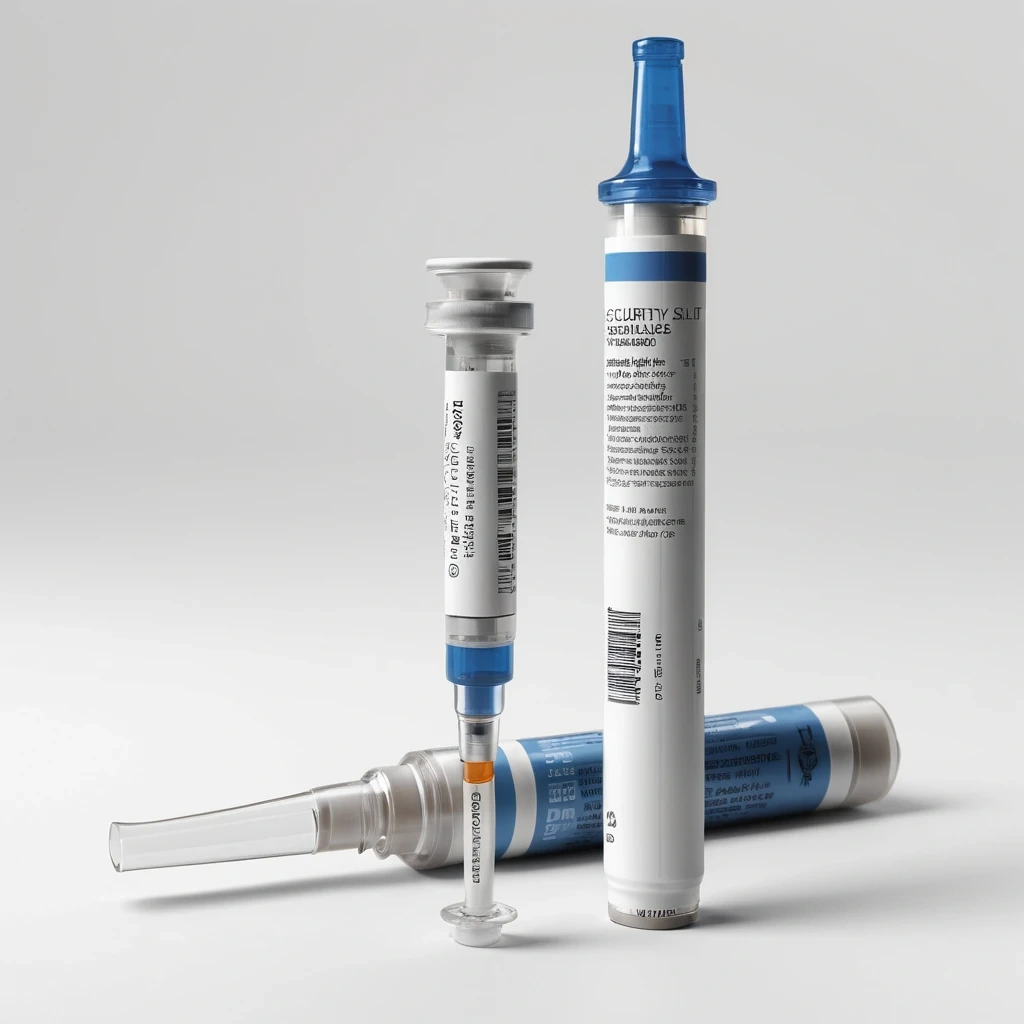

Medication integrity is critical in hospitals, especially when syringes are pre-drawn, transported, or stored temporarily. Without a tamper-evident seal, even slight movement of the cap can go unnoticed, creating risks in drug accuracy and patient safety. Standard labels don’t provide intrusion visibility and often peel when used across curved syringe surfaces. Anaika’s Tamper-Evident Syringe Wrap Labels are engineered with precision micro-perforations that break instantly if the cap is disturbed—delivering clear visual evidence of handling while maintaining strong adhesion and clean wrap-around performance.
Key Features
Tamper-evident micro-perforations reveal any cap movement or interference.
High-tack adhesive ensures secure bond on curved syringe barrels and cap joints.
Durable film resists tearing except along engineered security slits.
Smudge-resistant topcoat suitable for handwritten or printed identifiers.
Compatible with thermal transfer, digital, and laser printing systems.
Works with various syringe capacities (1 ml–20 ml).
Maintains bond strength during refrigeration and transport handling.
Technical Specifications
| Parameter | Details |
|---|---|
| Material | Synthetic tamper-evident film |
| Perforation Type | Micro-slit tamper indicators |
| Adhesive | High-tack medical-grade acrylic |
| Temperature Range | –10°C to +60°C |
| Print Compatibility | Thermal Transfer, Digital, Laser |
| Film Thickness | 50–70 microns |
| Security Feature | Breaks instantly on attempted removal |
| Shelf Life | 12 months |
Applications
Pre-drawn medication syringes
Critical care and anaesthesia workflows
Procedure rooms and operating theatres
Transport and temporary storage of prepared syringes
Pharmacy compounding units
Audit-driven medication safety programs
Why Choose Anaika?
Tamper-evident engineering designed for clear intrusion detection.
Films and adhesives tested on real syringe barrels for reliable wrap performance.
Custom perforation patterns and label dimensions available.
Consistent batch quality ensures predictable break strength and adhesion.
Resistant to disinfectants and frequent handling in clinical workflows.
Technical guidance to integrate labeling into medication safety protocols.

